MO:RE has initiated a new collaboration with Revvity, a developer and provider of end-to-end solutions designed to help scientists, researchers, and clinicians solve the world’s greatest health challenges. The partnership brings together our automated cell culture platform (MO:BOT) with Revvity’s assay development and detection capabilities, with the shared goal of improving standardization and reproducibility in 3D cell model-based applications, such as drug discovery and toxicity testing.
Addressing variability in 3D model research
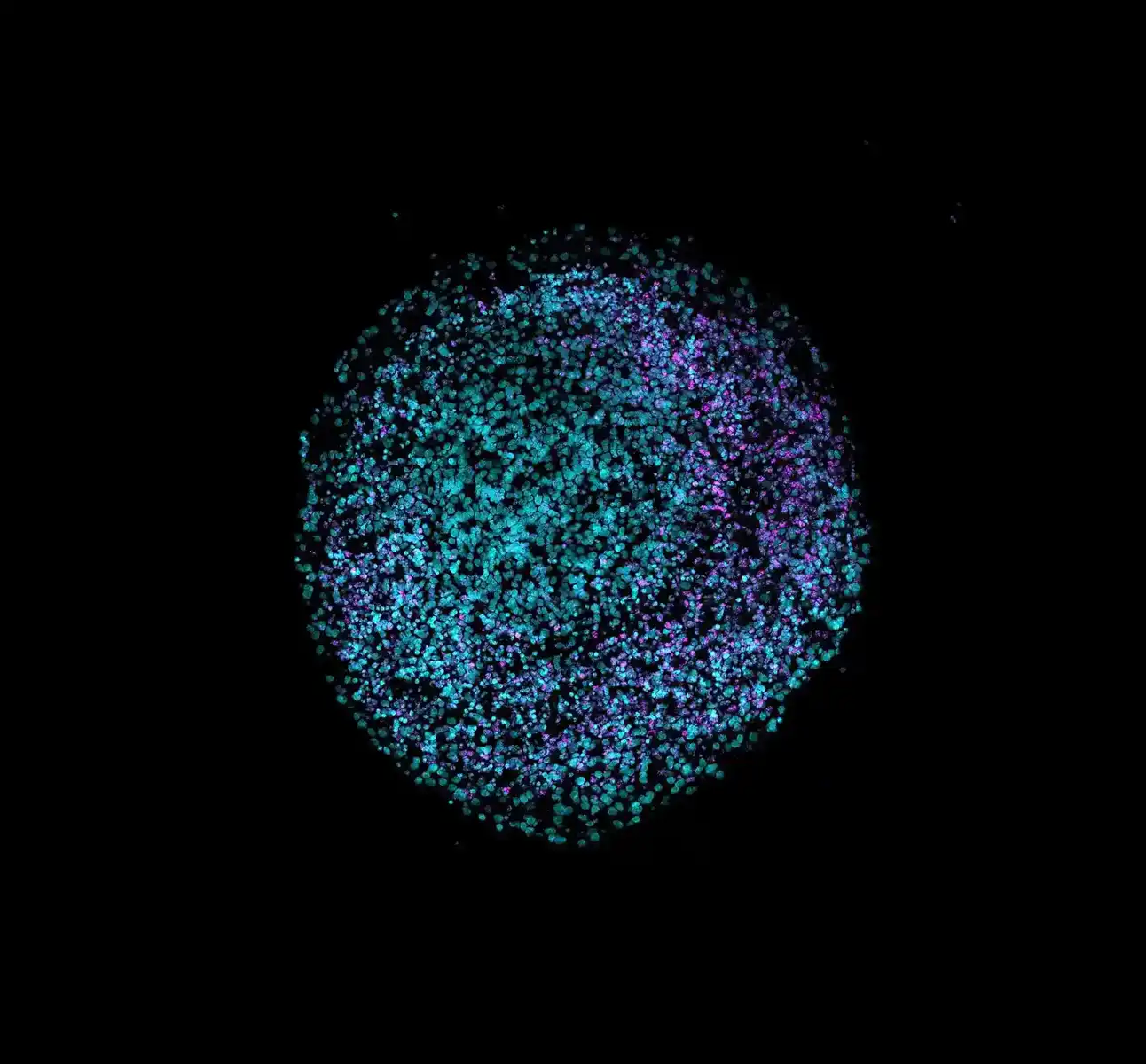
Organoids are advanced, miniaturized 3D cell culture systems that replicate key structural and functional features of human tissues. These physiologically relevant models enable researchers to study organ development, disease mechanisms, drug responses, and toxicity testing with greater accuracy compared to traditional two-dimensional systems. Additionally, they support the adoption of new approach methodologies (NAMs), which aim to reduce the reliance on animal models in preclinical studies.
One major challenge in organoid research is the variability in experimental data. Factors such as manual handling, inconsistent culturing conditions, and complex assay protocols can lead to data discrepancies, reducing reproducibility across laboratories and undermining the reliability of results.
The collaboration between Revvity and MO:RE seeks to directly address this challenge by leveraging automation to standardize workflows and optimize assay protocols, demonstrating how integrated automation can streamline and simplify assay development, even for advanced 3D model systems.
Collaborating to enhance reproducibility in organoid workflows
As a first step, we will generate liver spheroids, such as HepG2, using MO:BOT automated platform to validate Revvity’s ATPlite ™ assay. After spheroid generation, the workflow will utilize the AssayMate™ automated liquid handling workstation, integrated into an explorerTM G3 workstation, to automate an ATPlite 1glow-L assay protocol for toxicity testing in spheroids, with potential expansion to kidney organoids and other 3D cell models.
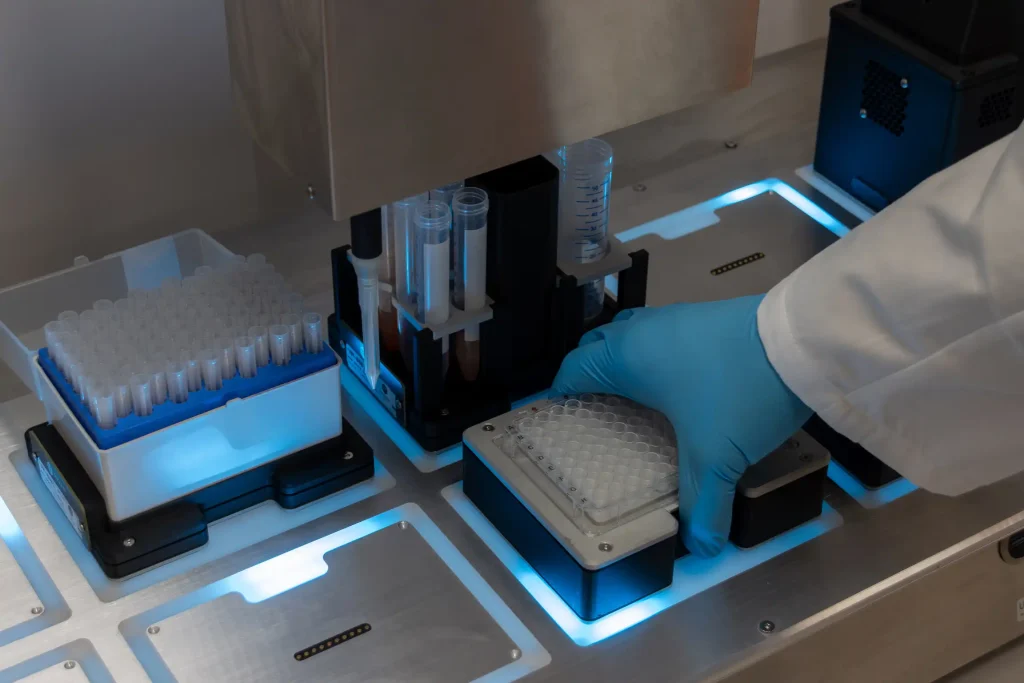
The MO:BOT platform is designed to simplify advanced cell culture workflows by automating key process steps involved in spheroid and organoid generation. By combining MO:BOT’s precise liquid handling capabilities for spheroid formation and culturing with Revvity’s ATPlite assay for cell viability analysis using Revvity plate readers, the collaboration aims to develop a streamlined, automation-driven workflow that enhances efficiency and reproducibility.
About the MO:BOT
The MO:BOT is a smart platform to automate, scale, and standardize 3D cell culture production for next-generation drug discovery and disease modeling.
MO:BOT integrates robotics, imaging, and validated biological protocols to automate cell seeding, media exchange, imaging, and data acquisition. This approach minimizes manual variability, ensures traceable experimental conditions, and facilitates large-scale parallel testing of multiple culture parameters. The platform has been designed with a modular approach, without cables or screws. Modules for imaging, heating, cooling, titing, or shaking can be arranged freely, and the software automatically recognizes each one to ensure seamless integration.
Future outcomes and broader applications
Organoids are transforming research by providing more accurate models of human biology. With their ability to mimic tissue complexity, they are helping researchers to uncover new insights into diseases and drug responses that might have been missed by traditional methods, while also reducing the reliance on animal models in preclinical research.
Through this collaboration, Revvity and MO:RE are addressing the challenge of data variation by integrating automated spheroid generation with robust viability assessment. The partnership plans to expand further by expanding validation efforts and integrating additional assay and imaging technologies.