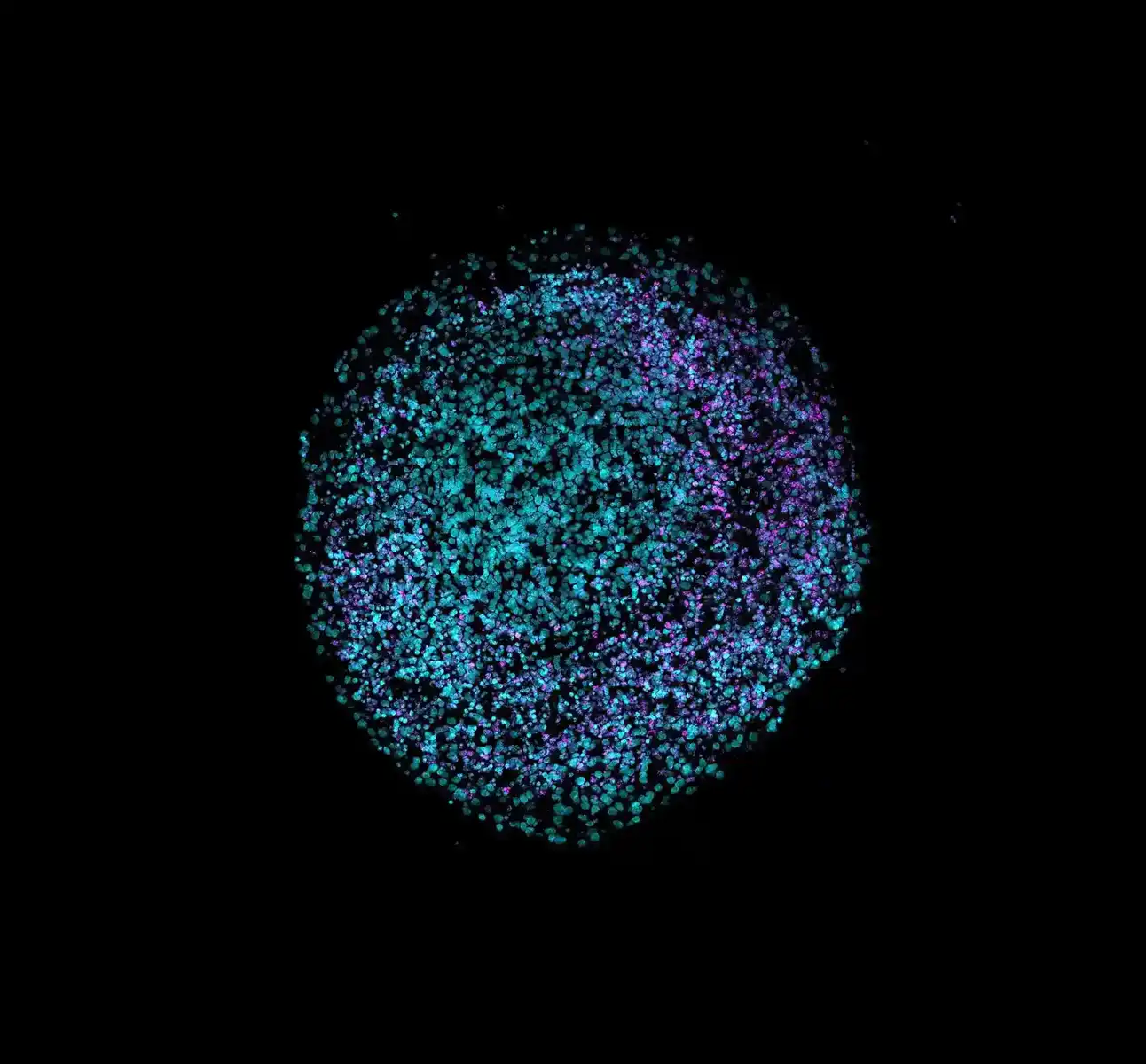
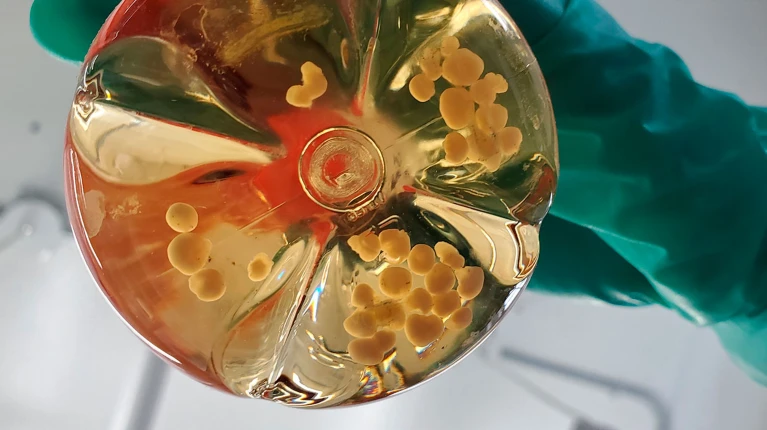
For decades, animal testing was the default gateway to human clinical trials. But that paradigm is rapidly evolving. Technologies known as new approach methodologies (NAMs) are no longer experimental side projects; they’re redefining how we assess drug safety and efficacy.
One compelling example comes from Stanford’s research on Timothy syndrome, where scientists used patient-derived brain organoids and assembloids to identify an antisense oligonucleotide that corrects the underlying genetic mutation. The team is now actively identifying patients worldwide to advance toward clinical trials. These breakthroughs are reinforced by regulatory momentum, such as the FDA Modernization Act 2.0, which paves the way for drug development grounded in human biology rather than animal models.
Can Drug Development Happen Without Animal Testing? Scientific and Regulatory Insights
Drug development without animal testing was once viewed as a distant ideal, but scientific advances in NAMs and recent regulatory changes make this vision plausible. The FDA Modernization Act 2.0, enacted in 2022, formally removed the statutory mandate for animal testing, making the use of non-animal data optional for drug applications. In April 2025, the FDA announced a plan to “reduce, refine, or potentially replace” animal testing for monoclonal antibodies and other drugs, encouraging manufacturers to submit NAMs data, such as human organoid assays and AI simulations, during Investigational New Drug (IND) submissions.
The European Medicines Agency (EMA) also fosters the regulatory acceptance of NAMs through several initiatives: it offers early, free dialogue with developers via the Innovation Task Force, formal scientific advice through the Scientific Advice Working Party, and qualification pathways that can result in opinions or letters of support. It also allows voluntary data submission in a safe harbour environment, reviews and updates guidelines to incorporate 3Rs-aligned alternatives, fosters international cooperation to harmonize criteria, and contributes to strategic documents that facilitate the integration of these methods into regulation. It has all been included in the website page dedicated to that matter.
This represents a regulatory sea‑change: by accepting NAMs, the FDA acknowledges that animal models often fail to predict human outcomes, and that human‑based methods may offer superior predictivity and efficiency.

The decision to pivot towards NAMS is not arbitrary. Only 1 out of 10 potential drugs tested in animals succeeds in clinical trials and is finally approved. Physiological and genetic differences between species limit the reliability of animal models. Adverse reactions in humans are frequently not detected in animal tests, while drugs deemed toxic in animals may be safe and effective in people, losing potential drugs that could save thousands of lives.
While full replacement of animal testing is not immediate, the combination of regulatory flexibility and scientific innovation in human-relevant models shows that drug development can, in time, rely primarily on non‑animal methods.
Technologies Replacing Animal Testing: From Organoids to AI Models
The transition to animal-free research hinges on advanced technologies that replicate human biology more accurately than traditional animal models:
- 3D cell culture: It encompasses models like spheroids and organoids. Spheroids are 3D clusters of cells grown in vitro that self-assemble into a spherical shape, typically formed from one or a few cell types, and are mainly used to study cell–cell interactions, gradients of nutrients and oxygen, or tumor-like growth. Organoids, in contrast, are derived from stem cells and self-organize into complex structures containing multiple cell types, mimicking the architecture and function of specific human organs, such as the liver, brain, or gut, making them powerful tools for disease modelling, drug testing, and regenerative medicine.
- Organ‑on‑a‑chip technology: Miniaturized systems that replicate the microenvironment and functions of human organs, consisting of a transparent microfluidic chip with channels lined by human cells growing and differentiating in a controlled microenvironment.
- Artificial intelligence (AI) and computational modeling: AI algorithms can simulate how drugs distribute, bind, and produce side effects in the human body, using just molecular and existing human data. This approach allows insights into pharmacokinetics and toxicity without animal exposure.
These non-animal testing platforms not only help reduce animal suffering, but in many cases, they detect toxicities earlier than animal studies could, offering both safety and predictive power.
How the FDA Is Transitioning Towards Animal-Free Drug Approvals
While the FDA Modernization Act 2.0 is a broad legal framework that removes the mandatory requirement for animal testing for all new drugs, the April 2025 directive on monoclonal antibodies is a roadmap. Producers of monoclonal antibodies may now submit NAMs data in IND filings and could qualify for streamlined reviews if their non‑animal data is strong. FDA animal testing alternatives include organoids, organ-on-a-chip, AI, and computational modeling. The FDA also announced it will begin tapping into existing human safety data from other countries to support assessments, cutting redundant animal studies.
The roadmap outlines immediate prioritization of monoclonal antibodies, followed by gradual expansion into other treatments and chemical entities. To facilitate implementation, the FDA will coordinate with the National Institutes of Health (NIH), the National Toxicology Program, and the Department of Veterans Affairs, through the Interagency Committee on Validation of Alternative Methods (ICCVAM). In July 2025, the agency hosted a stakeholder workshop to refine its plans and gather expert input. At this workshop, NIH Deputy Director of the Division of Program Coordination, Planning, and Strategic Initiatives (DPCPSI), Nicole Kleinstreuer, announced that the entity will stop accepting grant proposals focused exclusively on animal models.
The FDA has confirmed its intention to make animal‑free approaches not just optional, but preferable.
Challenges and Opportunities in Implementing Non-Animal Research Models
Despite clear progress, the shift toward non‑animal research does face hurdles. Validation remains a key challenge: NAMs must be rigorously assessed to ensure regulatory confidence in predicting human safety and efficacy. The ICCVAM Validation Workgroup prepared a document in 2024 to approach NAMs validation in toxicity testing, placing emphasis on integrating results from multiple in vitro and in chemico assays and in silico approaches.
Furthermore, scaling these technologies for widespread use requires significant investment in infrastructure and specialized training for scientists and regulators. International harmonization is also necessary to avoid fragmented standards that could discourage industry adoption.
Proposals are emerging to help democratize the use of NAMs. One of the most innovative examples is MO:BOT, the first fully automated platform that standardizes workflows in organoid drug screening with built-in quality control and regulatory-grade traceability. Every step from organoid generation to downstream assays is tracked, documented, and auditable, creating comprehensive data packages that support IND submissions and establish a foundation for GMP compliance. Unlike fragmented solutions, MO:BOT delivers an integrated system for producing, testing, and validating human-relevant models at scale.
By combining regulatory evolution, technological breakthroughs, and tools like MO:BOT, the industry has a unique opportunity to accelerate the adoption of non-animal models. This convergence promises not only a more ethical approach but also a more efficient, predictive, and economically viable animal-free drug development pipeline.
Sources
Cobb G. New NIH policy announced at joint FDA‑NIH workshop on reducing animal testing. FASEB Washington Update. 24 Jul 2025 [cited 2025 Aug 7]. Available from: https://www.faseb.org/journals-and-news/washington-update/new-nih-policy-announced-at-joint-fda-nih-workshop-on-reducing-animal-testing
European Coalition to End Animal Experiments. The future is animal‑free – accelerating humane and human‑relevant science. [Internet]. European Coalition to End Animal Experiments; 23 June 2025 [cited 2025 Aug 7]. Available from: https://www.eceae.org/arguments/the-future-is-animal-free-accelerating-humane-and-human-relevant-science#:~:text=Transitioning%20to%20animal%2Dfree%20research,saving%20potential%22%20(30).
European Medicines Agency. Regulatory acceptance of new approach methodologies (NAMs) to reduce animal use testing [Internet]. EMA; [cited 2025 Aug 7]. Available from: https://www.ema.europa.eu/en/human-regulatory-overview/research-development/ethical-use-animals-medicine-testing/regulatory-acceptance-new-approach-methodologies-nams-reduce-animal-use-testing
Goldman B, Digitale E. Brain organoids and assembloids are new models for elucidating, treating neurodevelopmental disorders. Stanford Medicine News Center. 2024 Apr 24 [cited 2025 Aug 7]. Available from: https://med.stanford.edu/news/all-news/2024/04/timothy-syndrome.html
National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM). Validation & qualification of new methods (resources for test method developers). NIEHS/NTP [Internet]. Updated Jun 2 2025 [cited 2025 Aug 7]. Available from: https://ntp.niehs.nih.gov/whatwestudy/niceatm/resources-for-test-method-developers/submissions
U.S. Food and Drug Administration. FDA announces plan to phase out animal testing requirement for monoclonal antibodies and other drugs [Internet]. 10 Apr 2025 [cited 2025 Aug 7]. Available from: https://www.fda.gov/news-events/press-announcements/fda-announces-plan-phase-out-animal-testing-requirement-monoclonal-antibodies-and-other-drugs