With the release of the Modernization Act 2.0 in 2025, the FDA formally accelerated the phase-out of animal models for new drug applications, strongly encouraging the adoption of human-relevant alternatives. Among these, organoids stand out for their structural complexity and physiological relevance, offering responses that are much closer to those of human organs than those of traditional cell lines.
However, reproducibility remains a major bottleneck. Manual organoid culture—especially for complex models like cardiac organoids—introduces unavoidable variability during differentiation, maintenance, and medium exchange. Automation removes this drift.
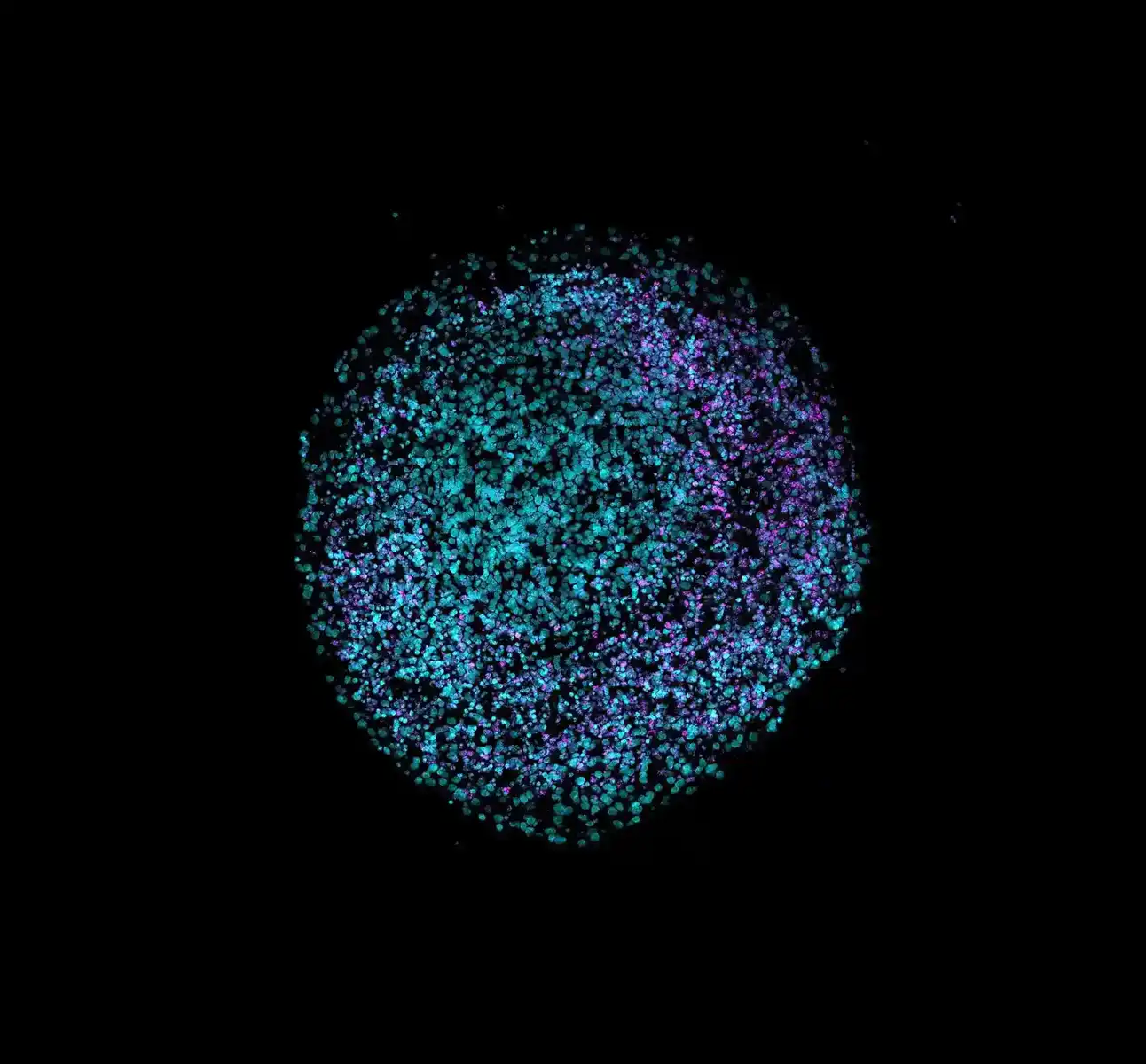
In their collaboration, ACROBiosystems and MO:RE integrate ACROBiosystems’ comprehensive organoid reagent portfolio into automated workflows. This includes cardiac organoid differentiation reagents, long-term maintenance, and endpoint characterization of validated antibodies for cardiac markers, all of which are also used in ACRO’s internal organoid development programs. The high batch-to-batch consistency of ACROBiosystems’ reagents further enables smooth automation by reducing variability and ensuring reliable performance across automated culture cycles.
The MO:BOT, MO:RE’s automated cell culture platform, aims to scale and standardize organoid culture for next-generation drug discovery and disease modeling. It integrates robotics, imaging, and validated biological protocols, minimizing technology friction thanks to its modular approach. Modules for imaging, heating, cooling, tilting, or shaking can be arranged freely, with no cables or screws, and the software automatically recognizes each one to ensure seamless integration. Cell seeding, media exchange, imaging, and data acquisition are completely automated.
Together, ACROBiosystems and MO:RE enable end-to-end, standardized cardiac organoid generation, characterization, and downstream applications.
The impact is clear: We generate more homogenous 3D cell aggregates and increase organoid viability across different human 3D in vitro models. As cardiotoxicity testing is a prerequisite for clinical studies, such robust, automated cardiac organoid systems are becoming foundational to next-generation, non-animal drug development.