Regulatory agencies are accelerating the shift toward non-animal preclinical testing, promoting New Approach Methodologies (NAMs) that better predict human outcomes. With the Modernization Act 2.0 and new initiatives to phase out animal testing, organoid 3D culture is in the spotlight as a human-relevant model capable of generating regulatory-grade data. Its potential, however, depends on overcoming current limitations in standardization, scalability, and traceability.
Why Standardization Matters in Organoid 3D Cultures for Drug Discovery
Organoid assays offer superior biological relevance compared to animal models, but their complexity and inherent variability can limit reliability. Without harmonized protocols, comparisons between experiments or institutions become unreliable, and regulatory agencies require reproducibility to accept data. Clear definitions of the context of use and robust quality control (QC) standards are essential to perform assays that are interpretable, reproducible, and acceptable. Standardization is therefore a prerequisite for generating valid, comparable data. When organoids are produced under consistent conditions, researchers can distinguish true drug responses from artefacts, reducing failure rates in later stages of drug development, lowering costs, and accelerating timelines.
Key Benefits of Automating Organoid Assays in Preclinical Research
Automation transforms labor-intensive organoid workflows into scalable and reproducible platforms. It minimizes human error, increases throughput, and ensures consistency across experiments. Automated systems integrate critical steps, seeding, media exchange, compound dosing, imaging, and data collection, in a controlled and repeatable manner.

Manual handling remains one of the main contributors to organoid development variability. Pipetting errors can damage or aspirate organoids, resulting in losses and inconsistent results. Evidence from collaborative studies between Mo:re and Organotherapeutics shows that automated medium exchanges preserve organoid morphology and enhance cell viability compared to manual handling. After three medium exchanges, manually cultured midbrain organoids lost over 15% of their area, while those maintained using the automated MO:BOT platform showed a loss of under 5% (Figure 1a). ATP levels, a proxy for cell viability, were significantly higher in organoids cultured with the Mo:re platform, confirming improved culture quality (Figure 1b).
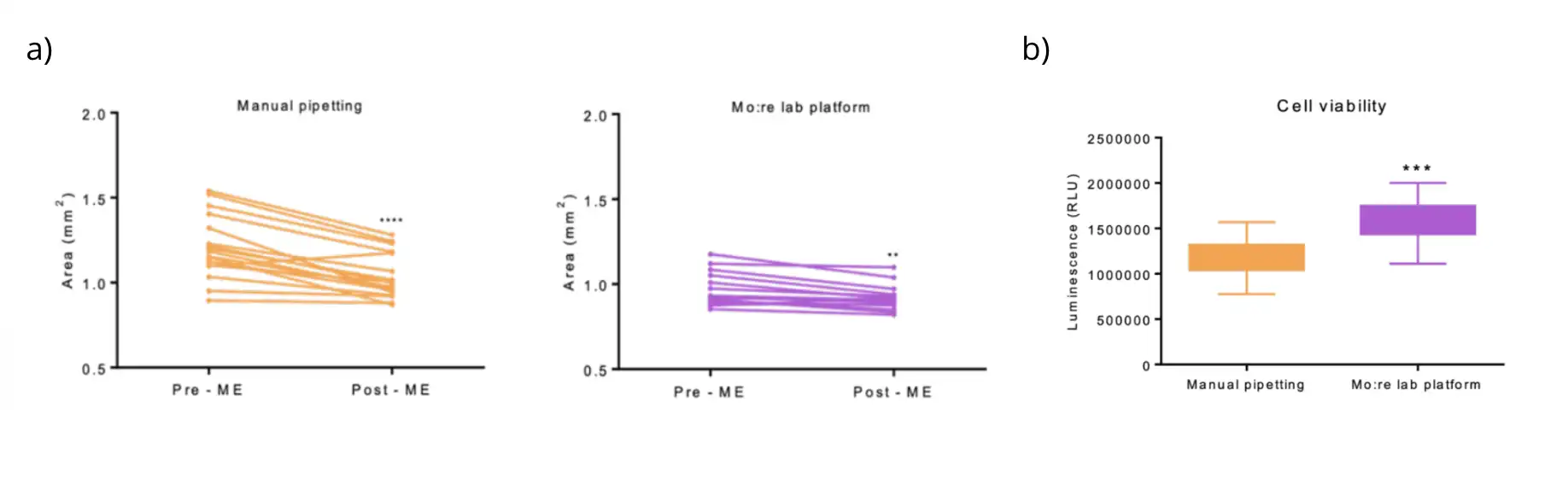
Figure 1. a) Midbrain organoids area (values expressed in mm2) before and after the first medium exchange performed by the Mo:re lab platform and manually. Data are shown for Pre-ME and Post-ME, respectively. P-value < 0.05. b) ATP level of midbrain organoids cultured manually and with the Mo:re lab platform, represented as RLU (Relative Luminescence Unit).RLU level of each organoid was normalized by its area. Values are presented as mean ±SD. P-value < 0.05.
Greater standardization increases the statistical power of assays, allowing either to use fewer samples to achieve significance or test more conditions in parallel. Moreover, automation ensures that every step is registered, creating a continuous, auditable record of the entire workflow. Automated, verified, and fully traceable organoid workflows, from culture to downstream assays, produce data packages that meet the stringent traceability and quality standards required by regulatory agencies. This capability turns organoid technologies into scalable, compliant tools for preclinical research and drug discovery.
Regulatory Considerations: From Quality Control to Data Traceability
The FDA Modernization Act 2.0 (2022) eliminated the legal mandate requiring animal testing for new drugs, opening the door to regulatory acceptance of non-animal methods, including organoids, microphysiological systems, and computer models. However, the act does not provide specific protocols, leaving the responsibility on developers to demonstrate that their methods are scientifically valid and reproducible.
Several initiatives are shaping the regulatory landscape to address this gap. The Ministry of Food and Drug Safety of South Korea, in partnership with Sungkyunkwan University, has inaugurated the Organoid Standards Initiative (OSI) and begun developing comprehensive guidelines in 2023 to facilitate standardized manufacturing and quality assessment of organoids. Additionally, the ISO Technical Committee 276 is developing ISO/AWI 25430‑2, an international standard that will define manufacturing, QC, and non-clinical use criteria for human organoids. Although still in draft form, it offers a credible framework for generating regulatory-grade data.
Automated systems align naturally with these requirements by generating digital audit trails, logging every step from cell sourcing to endpoint assays. This traceability supports compliance with 21 CFR Part 11 and facilitates the production of regulatory-grade data packages for IND submissions. Furthermore, agencies like the European Medicines Agency (EMA) are actively developing frameworks to assess the regulatory acceptance of New Approach Methodologies (NAMs), reinforcing the role of automated, standardized workflows.
Overcoming Technical Barriers in Organoid Culture Automation
Despite rapid advancements, technical challenges persist. Current hurdles include heterogeneity in cell sources, batch-to-batch variability in culture matrices, and the need for scalable imaging and AI-driven analysis pipelines. Traditional extracellular matrices (ECMs) such as Matrigel are animal-derived, poorly defined, and difficult to standardize. Emerging synthetic matrices, including PEG- and fibrin-based hydrogels, offer improved tunability and reproducibility but still require extensive validation.
Additionally, standardized QC parameters, such as organoid size, circularity, and viability, must be complemented by functional assays to capture the full complexity of organoid responses. Integrating these technologies into GMP-compliant frameworks remains a critical step toward regulatory approval.
The market is responding to these challenges with solutions designed to standardize and automate organoid cell culture. MO:BOT exemplifies this progress: it performs 3D cell culture in a fully automated way, supported by a software suite that simplifies experiment design, execution, and analysis. Every stage, from organoid generation to downstream assays, is tracked and auditable, producing comprehensive data packages that support IND submissions and establish a foundation for GMP compliance.
Standardization and automation are essential for achieving regulatory acceptance of organoids in drug screening. Initiatives like the OSI guidelines, the forthcoming ISO/AWI 25430‑2 standard, and frameworks aligned with the FDA Modernization Act 2.0 are moving organoids toward large-scale, regulatory-approved use. By combining validated protocols, automated workflows, and traceable data management, platforms such as MO:BOT are paving the way toward a new era of drug discovery; one where organoids replace animal testing, accelerating the development of safer, more effective therapies.
Sources
Ahn SJ, Lee S, Kwon D, Oh S, Park C, Jeon S, Lee JH, Kim TS, Oh IU. Essential Guidelines for Manufacturing and Application of Organoids. Int J Stem Cells. 2024 May 30;17(2):102-112. doi: 10.15283/ijsc24047.
Ahn SJ. Standards for Organoids. Int J Stem Cells. 2024 May 30;17(2):99-101. doi: 10.15283/ijsc24043.
Han JJ. FDA Modernization Act 2.0 allows for alternatives to animal testing. Artif Organs. 2023 Mar;47(3):449-450. doi: 10.1111/aor.14503.
ISO. ISO/AWI 25430‑2: Biotechnology — Organoids — Part 2: Manufacturing and quality control [Internet]. ISO; approved work item May 7 2025 [cited 2025 Aug 5]. Available from: https://www.iso.org/standard/91442.html
U.S. Food and Drug Administration. Part 11, electronic records; electronic signatures – scope and application [Internet]. FDA; [cited 2025 Aug 5]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/part-11-electronic-records-electronic-signatures-scope-and-application