Three-dimensional (3D) cell culture systems have emerged as a cornerstone in the transition toward new approach methodologies (NAMs), providing biologically relevant models that better replicate tissue architecture and cellular function. They address the limitations of traditional two-dimensional (2D) systems. Conventional monolayer cultures, while cost-effective and well standardized, do not reproduce the physiological architecture or the biochemical gradients that regulate cell behavior in vivo. These drawbacks limit their predictive potential in the preclinical setting.
3D Cell Culture Models: Principles and Key Applications
3D models recreate cell-cell and cell-matrix interactions, oxygen and nutrient gradients, and spatial organization, resulting in a more realistic representation of tissue behavior. They can be broadly divided into scaffold-free and scaffold-based systems. Scaffold-free approaches, such as spheroids, depend on spontaneous aggregation, while scaffold-based systems rely on hydrogels or biomaterials that mimic the extracellular matrix (ECM).
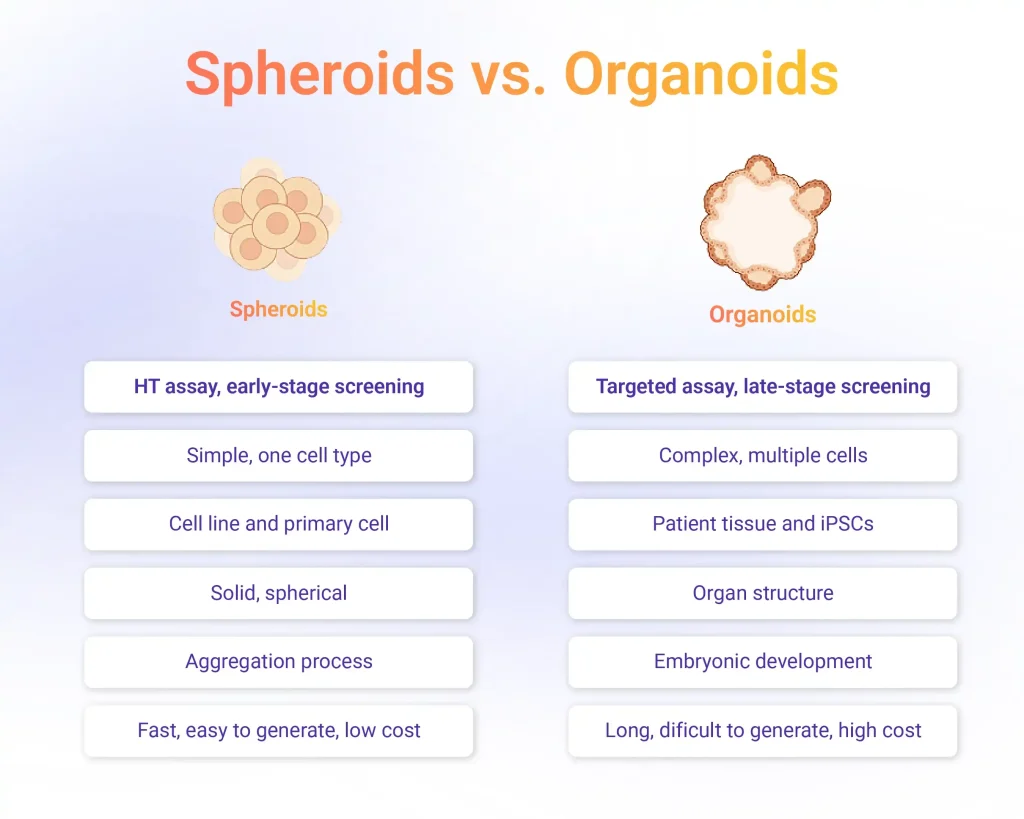
Spheroids
Spheroids are multicellular aggregates formed through spontaneous cell-cell adhesion, typically using non-adherent plates, hanging-drop methods, or bioreactors. Their structure allows the development of gradients for oxygen, nutrients, and metabolites, producing a heterogeneous population of proliferating, quiescent, and necrotic cells, conditions closer to those found in solid tissues or tumors.
Because of their simplicity and reproducibility, spheroids are widely used in early-phase toxicology screening and basic pharmacological studies. They are particularly useful for evaluating cell viability, cytotoxicity, and diffusion dynamics of drug candidates. Their microenvironmental gradients make them suitable for modeling tumor responses to chemotherapy and for assessing compound penetration and efficacy.
Organoids
Organoids represent a more complex level of 3D organization. They are derived from pluripotent or tissue-specific stem cells that self-organize into miniature, functional structures resembling real organs. This self-organization process depends on defined growth factors, signaling gradients, and extracellular matrix support.
Organoids reproduce the histological organization and genetic stability of the source tissue, allowing the study of organ development, pathology, and drug response. They have been applied to model organs such as the brain, intestine, liver, kidney, and lung, supporting research in developmental biology, cancer, and infectious diseases. Unlike spheroids, organoids exhibit cellular heterogeneity and structural compartmentalization, making them especially valuable for late-stage drug screening and precision medicine applications.
Organ-on-a-Chip Systems
Organ-on-a-chip (OOC) platforms take an extra step beyond, integrating microfluidics and 3D tissue engineering to simulate the physiological functions of organs. OOCs are engineered devices that use human cells, including spheroids or organoids, grown inside microfluidic channels to simulate key structures and physiological functions of an entire organ or organ system.
By incorporating continuous perfusion, mechanical stress, and inter-organ communication, these systems further improve the physiological relevance of in vitro assays. Their microarchitecture allows precise control over cell-environment interactions and offers opportunities for modeling systemic responses and multi-organ toxicity.

3D Cell Culture for Drug Screening and Toxicological Assessment
3D cell culture models have redefined how pharmacological and toxicological assays are designed, offering improved predictive power and reproducibility compared with 2D cultures. Their spatial organization and microenvironmental complexity support more realistic responses to drugs and environmental compounds.
These platforms support high-throughput testing of compound libraries, providing more predictive assessments of drug toxicity, efficacy, and metabolism. Tumor spheroids and patient-derived organoids form multicellular aggregates with heterogeneous zones of proliferation, quiescence, and apoptosis. This complexity allows accurate modeling of drug diffusion, penetration, and resistance patterns, which are essential for evaluating anticancer therapeutics.
A recent case that exemplifies organoid potential in drug discovery is the development of the bispecific antibody MCLA-158. From a library of more than 500 bispecific antibodies, functional screening across colorectal cancer organoids identified MCLA-158 as the most selective candidate. The entire discovery program relied exclusively on patient-derived colorectal cancer organoid lines, enabling direct assessment of tumor-specific responses. The selected antibody eliminated malignant organoids while leaving healthy colon organoids intact.
The program advanced from discovery to phase I trials in 30 months. Ongoing clinical trials include phase III studies in head and neck squamous cell carcinoma (NCT06496178) and a phase II trial in metastatic colorectal cancer (NCT03526835).
3D Models in Disease Modelling and Therapeutic Development
Organoids have become essential tools for studying human diseases and developing new therapies. By reproducing the structure, function, and cellular diversity of real tissues, they allow direct observation of human-specific mechanisms.
- In genetic and metabolic disorders, patient-derived or engineered organoids enable functional studies under physiological conditions. Intestinal organoids have been used to test CFTR modulators in cystic fibrosis, accurately predicting patient responses. Liver and kidney organoids reproduce key pathological traits of α1-antitrypsin deficiency, Wilson’s disease, and polycystic kidney disease, providing translational models for drug screening and validation.
- In neurological and infectious diseases modelling, brain organoids reveal mechanisms of neurodevelopmental and degenerative disorders such as Parkinson’s and Alzheimer’s disease, while also modeling viral infections like Zika or SARS-CoV-2. Similarly, intestinal and airway organoids are used to study viral entry, replication, and tissue-specific immune responses.
- In oncology, patient-derived tumor organoids retain the histology and mutational profile of primary tumors, enabling personalized drug testing and biomarker identification. Co-cultures incorporating immune and stromal cells allow the evaluation of immune checkpoint inhibitors and tumor-microenvironment interactions.
Emerging hybrid systems, such as assembloids and organoid-on-a-chip platforms, further enhance physiological relevance by integrating vascular, neural, and immune components in disease modelling using 3D cell culture systems.
Advantages and Challenges of 3D Cell Culture Systems
3D culture systems offer clear advantages over traditional 2D models, mainly by preserving the spatial organization, cell-cell communication, and physiological gradients that regulate tissue function. Their capacity to mimic native architecture enables more accurate assessment of drug responses, toxicity, and disease mechanisms. Spheroids provide scalability and cost efficiency for early-stage screening, while organoids deliver higher physiological fidelity for mechanistic and personalized research.
Despite their potential, from a biological perspective, one of the main limitations of current 3D models is the lack of vascularization. Most spheroids and organoids depend on passive diffusion of oxygen and nutrients, restricting their size and leading to necrotic regions in larger constructs. Recent approaches aim to overcome this by simultaneously differentiating epithelial and endothelial cells using carefully timed molecular cues. Using this approach, organoids derived from iPSCs can form vasculature from the earliest stages of development, although these kinds of organoids are not mature enough to act as real organs.
From the technical side, variability in matrix composition, growth media, and manual manipulation introduces batch-to-batch differences that compromise reproducibility across laboratories. Protocol diversity makes it difficult to standardize procedures, which in turn affects data comparability and regulatory acceptance. Scalability is another challenge, as organoid generation often requires long culture times, precise growth conditions, and high operator expertise. Integrating automated workflows, liquid handling, and quantitative imaging can mitigate these issues by reducing human error and ensuring consistency in experimental outcomes.
Platforms such as MO:BOT integrate robotics, imaging, and validated biological protocols to automate cell seeding, media exchange, image and data acquisition. This approach minimizes manual variability, ensures traceable experimental conditions, and facilitates large-scale parallel testing of multiple culture parameters.
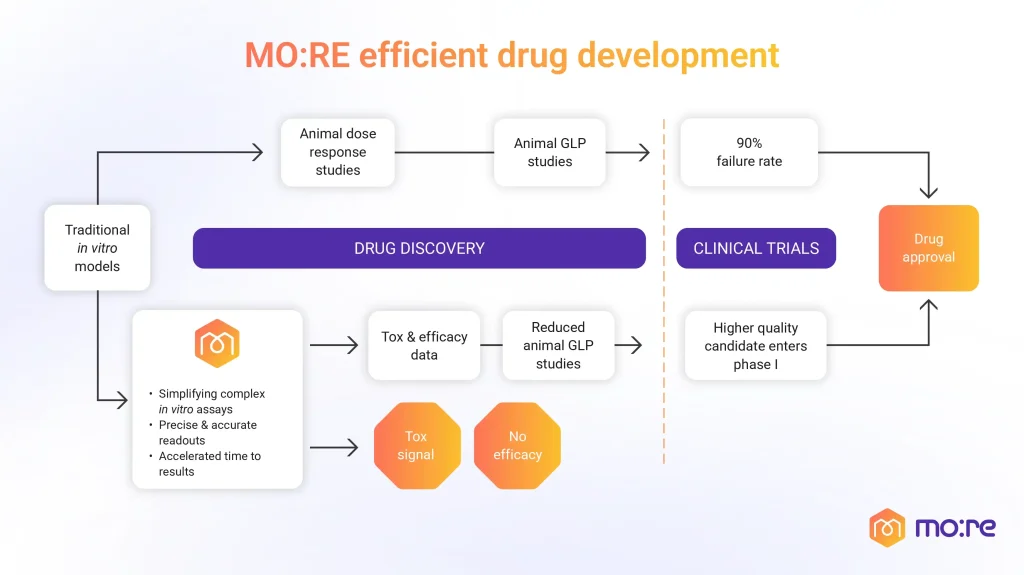
By combining automation with pre-verified organoid and spheroid protocols, MO:BOT supports reproducibility and scalability, two of the main technical bottlenecks in 3D culture research. These advances not only accelerate preclinical timelines but also align with the ethical and regulatory goals of NAMs, positioning automated 3D models as essential tools for the next generation of drug discovery and toxicological assessment.
Sources
Biju TS, Priya VV, Francis AP. Role of three-dimensional cell culture in therapeutics and diagnostics: an updated review. Drug Deliv Transl Res. 2023 Sep;13(9):2239-2253. doi: 10.1007/s13346-023-01327-6.
Dave R, Pandey K, Patel R, Gour N, Bhatia D. Leveraging 3D cell culture and AI technologies for next-generation drug discovery. Cell Biomat. 2025 Apr 22. 1(3). doi: 10.1016/j.celbio.2025.100050.
Herpers B, Eppink B, James MI, Cortina C, Cañellas-Socias A, Boj SF, Hernando-Momblona X, Glodzik D, Roovers RC, van de Wetering M, Bartelink-Clements C, Zondag-van der Zande V, Mateos JG, Yan K, Salinaro L, Basmeleh A, Fatrai S, Maussang D, Lammerts van Bueren JJ, Chicote I, Serna G, Cabellos L, Ramírez L, Nuciforo P, Salazar R, Santos C, Villanueva A, Stephan-Otto Attolini C, Sancho E, Palmer HG, Tabernero J, Stratton MR, de Kruif J, Logtenberg T, Clevers H, Price LS, Vries RGJ, Batlle E, Throsby M. Functional patient-derived organoid screenings identify MCLA-158 as a therapeutic EGFR × LGR5 bispecific antibody with efficacy in epithelial tumors. Nat Cancer. 2022 Apr;3(4):418-436. doi: 10.1038/s43018-022-00359-0.
Mallapaty S. Mini hearts, lungs and livers made in lab now grow their own blood vessels. Nature. 2025 Jul;643(8073):892. doi: 10.1038/d41586-025-02183-9.
Wang D, Villenave R, Stokar-Regenscheit N, Clevers H. Human organoids as 3D in vitro platforms for drug discovery: opportunities and challenges. Nat Rev Drug Discov. 2025. doi:10.1038/s41573-025-01317-y